การรักษาเส้นเลือดขอดด้วย เลเซอร์
การระเหยด้วยเลเซอร์แบบ Endovenous อยู่ภายใต้ชื่อกรรมสิทธิ์หลายประการ เช่น EVLT, ELVes, VeinSeal ขึ้นอยู่กับผู้ผลิตเลเซอร์ ในบทความนี้ เราใช้คำทั่วไปว่า EVLA สำหรับ Endovenous Laser Ablation เนื่องจากเลเซอร์ทุกประเภทจะเหมือนกันหมด
EVLA คืออะไร?
EVLA เป็นวิธีการใหม่ในการรักษาเส้นเลือดขอดโดยไม่ต้องผ่าตัด แทนที่จะมัดและเอาเส้นเลือดที่ผิดปกติออก พวกมันจะถูกทำให้ร้อนด้วยเลเซอร์ ความร้อนจะทำลายผนังของเส้นเลือดและร่างกายจะดูดซับเนื้อเยื่อที่ตายแล้วตามธรรมชาติและเส้นเลือดที่ผิดปกติจะถูกทำลาย สามารถทำได้ในห้องทรีตเมนต์ธรรมดาแทนที่จะเป็นห้องผ่าตัด
เส้นเลือดของฉันเหมาะสำหรับ EVLA หรือไม่?
ผู้ป่วยที่มีเส้นเลือดขอดเกือบทั้งหมดเหมาะสำหรับ EVLA ผู้ที่ไม่เหมาะ (โดยปกติคือผู้ที่มีเส้นเลือดขอดเล็ก ๆ หลังการผ่าตัดครั้งก่อน) สามารถจัดการได้ด้วยโฟม sclerotherapy
ขั้นตอนเกี่ยวข้องกับอะไร?
ทำการสแกนด้วยอัลตราซาวนด์และเส้นเลือดที่จะรักษาจะถูกทำเครื่องหมายด้วยปากกา คุณนอนบนโซฟาและขาของคุณสะอาดและคลุมด้วยผ้า คุณอาจอยู่ด้านหลังหรือด้านหน้าของคุณทั้งนี้ขึ้นอยู่กับเส้นเลือดที่ต้องการรักษา ขั้นตอนทั้งหมดเหล่านี้ได้รับคำแนะนำจากการสแกนด้วยอัลตราซาวนด์
Endovenous หมายถึงภายในเส้นเลือด ดังนั้นสิ่งต่อไปที่แพทย์ต้องทำคือการเข้าไปในเส้นเลือดของคุณ ยาชาเฉพาะที่จำนวนเล็กน้อยถูกฉีดเข้าไปในผิวหนังเหนือหลอดเลือดดำและสอดเข็มเข้าไป ลวดถูกส่งผ่านเข็มขึ้นไปบนเส้นเลือด เข็มจะถูกลบออกและสอดสายสวน (ท่อพลาสติกแบบบาง) ผ่านเส้นลวด ขึ้นหลอดเลือดดำและดึงลวดออก
เส้นใยเลเซอร์จะถูกส่งผ่านไปยังสายสวน ดังนั้นปลายของเส้นใยจึงอยู่ที่จุดสูงสุดเพื่อให้ความร้อน (โดยปกติคือบริเวณขาหนีบ) จากนั้นฉีดยาชาเฉพาะที่ปริมาณมากรอบๆ เส้นเลือดโดยใช้เข็มเล็กๆ หลายๆ เข็ม พนักงานและผู้ป่วยทุกคนใส่ข้อกำหนดด้านความปลอดภัยของเลเซอร์เพื่อเป็นการป้องกันไว้ก่อน จากนั้นเลเซอร์จะยิงขึ้นและดึงหลอดเลือดดำลงมาประมาณ 5 นาที คุณจะได้ยินเสียงเตือนดังขึ้น และอาจมีกลิ่นหรือรสแสบร้อนแต่จะไม่รู้สึกเจ็บปวดใดๆ หากคุณรักษาขาทั้งสองข้าง ให้ทำซ้ำที่ขาอีกข้างหนึ่ง เลเซอร์และสายสวนจะถูกลบออกและเข็มเจาะปกคลุมด้วยน้ำสลัดขนาดเล็ก
การรักษาใช้เวลาประมาณ 20-30 นาทีต่อขา คุณอาจได้รับโฟม sclerotherapy หรือการทำ avulsions แล้วใส่ถุงน่องแบบบีบอัด
จะเกิดอะไรขึ้นหลังการรักษา?
ไม่นานหลังจากการรักษาของคุณ คุณจะได้รับอนุญาตให้กลับบ้าน ไม่แนะนำให้ขับรถแต่ใช้ระบบขนส่งสาธารณะ เดินหรือให้เพื่อนขับ คุณจะต้องสวมถุงน่องนานถึงสองสัปดาห์ และคุณจะได้รับคำแนะนำเกี่ยวกับวิธีการอาบน้ำ คุณควรจะสามารถกลับไปทำงานได้ทันทีและทำกิจกรรมตามปกติได้มากที่สุด
คุณไม่สามารถว่ายน้ำหรือทำให้ขาเปียกในช่วงเวลาที่แนะนำให้คุณสวมถุงน่อง ผู้ป่วยส่วนใหญ่จะรู้สึกตึงตามความยาวของหลอดเลือดดำที่รับการรักษา และบางคนอาจมีอาการปวดบริเวณนั้นประมาณ 5 วันต่อมา แต่อาการนี้มักไม่รุนแรง ยาแก้อักเสบทั่วไปอย่างไอบูโพรเฟนก็เพียงพอแล้วที่จะบรรเทาได้
เลเซอร์ทั้งหมดเหมือนกันหรือไม่?
เลเซอร์ส่วนใหญ่ที่ใช้สำหรับ EVLA เกือบจะเหมือนกัน บางประเภทใหม่อ้างว่าทำให้เกิดความเจ็บปวดน้อยกว่าเลเซอร์มาตรฐาน ดูเหมือนว่าจะมีความจริงบางอย่างในเรื่องนี้แม้ว่าระดับความเจ็บปวดที่ผู้ป่วยส่วนใหญ่ที่ใช้เลเซอร์มาตรฐานจะพบจะมีน้อยและยอมรับได้ง่าย เลเซอร์ใหม่นี้ไม่ได้ผ่านการทดสอบทางคลินิกอย่างครอบคลุมแบบเดียวกัน ดังนั้นเราจึงไม่ทราบแน่ชัดว่าเลเซอร์จะได้ผลหรือไม่ โดยเฉพาะอย่างยิ่งในระยะยาว
ฉันจะต้องได้รับการรักษาเพิ่มเติมหรือไม่?
หากคุณกำลังรับการรักษาเพียงเพื่อบรรเทาอาการ โดยปกติไม่จำเป็นต้องทำการรักษาเพิ่มเติม อย่างไรก็ตาม ผู้ป่วยส่วนใหญ่ต้องการที่จะปรับปรุงลักษณะที่ปรากฏของเส้นเลือดของพวกเขา และในจำนวนนี้ประมาณ 80% จะต้องได้รับการรักษาเพิ่มเติม อาการเส้นเลือดขอดมักจะไม่ชัดเจนหลังจาก EVLA แต่ไม่ค่อยหายไปอย่างสมบูรณ์
การรักษาเพิ่มเติมสำหรับเส้นเลือดขอดสามารถเป็นได้ทั้งโดยการฉีดหรือโฟม sclerotherapy การรักษาเพิ่มเติมเหล่านี้สามารถทำได้ในช่วงเวลาของ EVLA หรือนานกว่านั้นโดยปกติหลังจากล่าช้าไป 4-6 สัปดาห์ หากคุณมีเส้นเลือดขอดที่ขาทั้งสองข้าง เป็นไปได้ยากมากที่คุณจะสามารถทำการรักษาเพิ่มเติมทั้งหมดได้ในขณะที่ทำ EVLA
การถอนพิษจะดำเนินการหลังจากฉีดยาชาเฉพาะที่รอบๆ เส้นเลือดเพื่อทำให้บริเวณนั้นชา แผลเล็ก ๆ ทำขึ้นเหนือเส้นเลือดและถูกแหย่ด้วยเข็มควัก คุณอาจต้องกรีดเล็กๆ หลายๆ แผล แต่จะหายได้ง่ายโดยไม่ต้องเย็บแผลและมีแผลเป็นน้อยที่สุด
การรักษาด้วยโฟม sclerotherapy เป็นวิธีที่พบได้บ่อยที่สุดในการจัดการกับเส้นเลือดขอดที่ตกค้างหลังจาก EVLA และมีประสิทธิภาพสูงสำหรับสิ่งเหล่านี้
ภาวะแทรกซ้อนคืออะไร?
ภาวะแทรกซ้อนที่ร้ายแรงหลังจาก EVLA นั้นหายากมาก
Thrombophlebitis: EVLA ทำงานโดยการให้ความร้อนแก่ผนังหลอดเลือดดำและการตอบสนองต่อความร้อนโดยเจตนาอย่างหลีกเลี่ยงไม่ได้คือการอักเสบของผนังหลอดเลือดดำ คุณอาจรู้สึกว่าเส้นเลือดที่รับการรักษาแข็งและอ่อนโยน เส้นเลือดขอดที่ป้อนจากหลอดเลือดดำที่รักษาอาจแข็งและเป็นก้อนเนื่องจากลิ่มเลือดก่อตัวขึ้นภายในเส้นเลือด ลิ่มเลือดนี้ไม่เป็นอันตราย และร่างกายของคุณจะดูดซึมไปตามธรรมชาติภายในเวลาไม่กี่สัปดาห์
ความเสียหายของเส้นประสาท: เนื่องจากเส้นประสาทสามารถอยู่ข้างเส้นเลือด สิ่งเหล่านี้อาจได้รับความเสียหายจากความร้อนหรือการอาเจียน และผู้ป่วยบางรายจะสังเกตเห็นอาการชาเล็กน้อยบนผิวหนัง สิ่งเหล่านี้มักจะแก้ไขได้ภายในสองสามเดือน
แผลไหม้: แม้ว่าจะเป็นไปได้ที่จะเผาผิวหนังด้วยเลเซอร์ในทางปฏิบัติ แต่ก็หายากมาก
DVT: A DVT เป็นลิ่มเลือดในหลอดเลือดดำส่วนลึกที่ขา เป็นภาวะแทรกซ้อนที่ได้รับการยอมรับจากการผ่าตัดและอาจเป็นอันตรายได้หากลิ่มเลือดแตกออกและเดินทางไปยังปอด ความเสี่ยงในการได้รับ DVT หลังจาก EVLA นั้นต่ำมาก แต่มีรายงานแล้ว
ขั้นตอนใดที่เหมาะกับฉัน
ผู้ป่วยจำนวนมากที่พิจารณา EVLA สับสนว่าควรรักษาแบบใด เรื่องนี้ไม่น่าแปลกใจ เนื่องจากมีหลายทางเลือก รวมถึงการไม่ทำอะไรเลย การผ่าตัด และการรักษาที่ไม่ผ่าตัด เช่น Foam Sclerotherapy, RFA (VNUS) และ EVLA
มากจะขึ้นอยู่กับประสบการณ์ของผู้เชี่ยวชาญด้านหลอดเลือดของคุณ ศัลยแพทย์บางคนยังคงทำการผ่าตัดเท่านั้น แต่ปัจจุบันส่วนใหญ่มีทางเลือกในการรักษาใหม่อย่างน้อยหนึ่งทาง การไม่ทำอะไรเลยเป็นทางเลือกที่ดีถ้าคุณไม่ใส่ใจกับลักษณะที่ปรากฏของเส้นเลือดและไม่ก่อให้เกิดอาการสำคัญ ไม่จำเป็นต้องรักษาเส้นเลือดขอดส่วนใหญ่
การสวมถุงน่องเป็นทางเลือกที่ดีสำหรับผู้ที่มีอาการแต่ไม่กังวลเกี่ยวกับลักษณะของขาและเต็มใจที่จะสวมถุงน่องไปตลอดชีวิต
ผู้ป่วยบางรายไม่จำเป็นต้องทำการผ่าตัดแต่ก็ยังเป็นที่ต้องการของคนไข้ โดยเฉพาะผู้ที่ต้องการให้ยาสลบ ผู้ที่ไม่ชอบฉีดยา และผู้ที่มีเส้นเลือดขอดที่ขาทั้งสองข้างที่ต้องการการรักษาทั้งหมดในคราวเดียว
หากคุณต้องการการรักษาขั้นสุดท้ายแต่ต้องการหลีกเลี่ยงการผ่าตัดและการดมยาสลบและกลับสู่กิจกรรมปกติ
Endovenous laser ablation goes under several proprietary names e.g. EVLT, ELVes, VeinSeal, depending on the manufacturer of the laser. In this article we use the general term EVLA for Endovenous Laser Ablation, as all the various types of laser ablation are essentially the same.
What is EVLA?
EVLA is a new method of treating varicose veins without surgery. Instead of tying and removing the abnormal vein,s they are heated by a laser. The heat kills the walls of the veins and the body then naturally absorbs the dead tissue and the abnormal veins are destroyed. It can be carried out in a simple treatment room rather than an operating theatre.
Are my veins suitable for EVLA?
Almost all patients with varicose veins are suitable for EVLA. Those few who are not suitable (usually those with small recurrent veins after previous surgery) can usually be managed with just foam sclerotherapy.
What does the procedure involve?
An ultrasound scan is performed and the veins to be treated are marked with a pen. You lie on a couch and your leg is cleaned and covered with drapes. Depending on which veins are to be treated, you may be on your back or your front. All these steps are guided by ultrasound scanning.
Endovenous means inside the vein, so the next thing the doctor has to do is to get inside your vein. A small amount of local anaesthetic is injected into the skin over the vein and a needle inserted into it. A wire is passed through the needle and up the vein. The needle is removed and a catheter (thin plastic tubing) is passed over the wire, up the vein and the wire removed.
A laser fibre is passed up the catheter so its tip lies at the highest point to be heated (usually your groin crease). A large quantity of local anaesthetic solution is then injected around the vein through multiple tiny needle pricks. All staff and the patient put on laser safety specs as a precaution. The laser is then fired up and pulled down the vein over about 5 minutes. You will hear a warning buzzer ringing and may smell or taste burning but won’t feel any pain. If you’re having both legs treated the process is repeated on the other leg. The laser and catheter are removed and the needle puncture covered with a small dressing.
The treatment takes about 20-30 minutes per leg. You may also have some foam sclerotherapy or some avulsions undertaken and a compression stocking is then put on.
What happens after treatment?
Soon after your treatment you will be allowed home. It is advisable not to drive but to take public transport, walk or have a friend drive you. You will have to wear the stockings for up to two weeks and you will be given instructions about how to bathe. You should be able to go back to work straight away and get on with most normal activities.
You cannot swim or get your legs wet during the period in which you have been advised to wear the stockings. Most patients experience a tightening sensation along the length of the treated vein and some get pain in that area around 5 days later but this is usually mild. Normal anti-inflammatory drugs like Ibuprofen are normally sufficient to relieve it.
Are all lasers the same?
Most of the lasers used for EVLA are almost identical. Some new types claim to cause less pain than the standard lasers. There does appear to be some truth in this, although the degree of pain experienced by most patients with standard lasers is minimal and easily tolerated. The new lasers have not undergone the same extensive clinical testing and therefore we don’t know for certain if they will be as effective, especially in the longer term.
Will I need further treatment?
If you are having treatment just to relieve symptoms then no further treatment is usually necessary. Most patients however wish also to improve the appearance of their veins and of these about 80% will require further treatment. The varicosities normally become less obvious after EVLA but rarely disappear completely.
Additional treatment for the varicosities can be either by avulsions or foam sclerotherapy. These additional treatments can be undertaken at the time of the EVLA or more usually after a delay of 4-6 weeks. If you have extensive varicose veins on both legs it is very unlikely that you will be able to have all the additional treatment undertaken at the time of the EVLA.
Avulsions are undertaken after local anaesthetic solution has been injected around the veins to numb the area. Small incisions are made over the veins and they are teased out with a crochet hook. You may require many small incisions but they heal easily without stitches and with minimal scarring.
Foam sclerotherapy is the commonest means of dealing with residual varicose veins after EVLA and is highly effective for these.
What are the complications?
Serious complications after EVLA are very rare.
Thrombophlebitis: EVLA works by heating the wall of the vein and an inevitable and deliberate response to the heat is an inflammation of the vein wall. You may feel the vein that has been treated become hard and tender. Varicose veins that feed from the treated vein may also become hard and lumpy as some blood clot forms within them. This clot is not dangerous and your body will naturally absorb it over a few weeks.
Nerve damage: As nerves can lie alongside the veins these may also become damaged by the heat or by avulsions and a few patients notice small patches of numbness on their skin. These usually resolve over a few months.
Burns: Although it is possible to burn the skin with the laser in practice this is very rare indeed.
DVT: A DVT is a blood clot in the deep veins in the leg. It is a recognised complication of surgery and can be dangerous if the clot breaks away and travels to the lungs. The risk of getting a DVT after EVLA is very low but has been reported.
Which procedure is right for me?
Many patients considering EVLA are confused as to which treatment to go for. This is unsurprising, as there are several options including doing nothing, having an operation and several new non-surgical treatments like Foam Sclerotherapy, RFA (VNUS) and EVLA.
Much will depend on the experience of your vascular specialist. Some surgeons still only undertake surgery but most now offer at least one of the new treatment options. Doing nothing is a good option if you are not bothered by the appearance of your veins and they are not causing significant symptoms. There is no medical need to treat most varicose veins.
Wearing stockings is a good option for those with symptoms but who are not worried about the appearance of their legs and are willing to wear stockings for the rest of their lives.
Surgery is rarely required but still preferred by some patients, especially those who wish to have a general anaesthetic, those who don’t like injections and those with extensive varicose veins on both legs who want all treatment carried out in one session.
If you want definitive treatment but want to avoid surgery and general anaesthesia and to get back to normal activities
Sclerotherapy treatment
quickly you should consider one of the following treatments:
- Foam sclerotherapy is good especially for those with less extensive veins who are willing to accept the possible need for several sessions of treatment and the possibility of the veins recurring in the future and further treatment becoming necessary. It is the least invasive option.
- RFA (VNUS, RFITT) is good for those with a long wide straight segment of vein which requires treating. Approximately 70% of patients are suitable for RFA.
- EVLA is suitable for almost all patients however short or wide their veins are.The method and results of treatment are almost identical with RFA and EVLA.
N.B. Foam sclerotherapy, RFA and EVLA all require at least two treatment sessions in most patients. Expect it to take at least 8 weeks to see the full effect of treatment. Look at the information about all the different options and ask your specialist’s advice before coming to a decision about which treatment is right for you.
Whilst we make every effort to ensure that the information contained on this site is accurate, it is not a substitute for medical advice or treatment, and the Circulation Foundation recommends consultation with your doctor or health care professional.
The Circulation Foundation cannot accept liability for any loss or damage resulting from any inaccuracy in this information or third party information such as information on websites to which we link.
The information provided is intended to support patients, not provide personal medical advice.
Chronic venous disorders (CVDs) of the lower extremity are common problems caused by venous hypertension, which is commonly the result of reflux in one or more of the saphenous veins and their primary tributaries. Treatment options in patients with saphenous vein incompetence include conservative management or elimination of these incompetent pathways using endovenous techniques or surgery.
Although conservative management with compression therapy may improve the symptoms of chronic venous insufficiency, it does not cure it. Historically, surgery was considered the best way to eliminate incompetence using ligation of the saphenous vein at its deep vein junction and removal of the abnormal saphenous vein segments; this procedure is known as high ligation and stripping (HL/S). Over the last 10-15 years, HL/S has been essentially replaced by percutaneous endovenous thermal ablation. Two types of thermal ablation procedures exist: endovenous laser ablation (ELA) and radiofrequency ablation (RFA). Both procedures are associated with high success and low complication rates. The procedures are generally performed on an ambulatory basis with local anesthetic and typically require no sedation. The patients are fully ambulatory following treatment, and the recovery time is short. In this article, ELA is reviewed in detail.
ELA mechanism of action
The underlying goal for all thermal ablation procedures is to deliver sufficient thermal energy to the wall of an incompetent vein segment to produce irreversible occlusion, fibrosis, and ultimately disappearance of the vein. The mechanism of vein wall injury after ELA is controversial. It has been postulated to be mediated both by direct effect and indirectly via laser-induced steam generated by the heating of small amounts of blood within the vein.[1] Adequately damaging the vein wall with thermal energy is imperative to obtain effective ablation. Some heating may occur by direct absorption of photon energy (radiation) by the vein wall, as well as by convection from steam bubbles and conduction from heated blood. However, these later mechanisms are unlikely to account for most of the impact on the vein.
Diode lasers are most commonly used for ELA. Laser generators exist with multiple different wavelengths, including lower wavelengths that are considered hemoglobin specific and include 810 nm, 940 nm, 980 nm, and 1064 nm. Higher wavelengths are considered water specific and include 1320 nm and 1470 nm. Although it is still not definitively established in the literature, some authors suggest that the higher wavelength lasers produce similar efficacy at lower power settings with less postprocedure symptoms.[2]
It can be performed with multiple different laser fiber designs (ie, bare-tip fibers, jacket-tip fibers [see image below], radial fibers) and diameters available from a variety of vendors. Each of the fiber designs has been demonstrated to be effective in closing the saphenous vein. At this point, there are no conclusive data demonstrating a superiority of a given fiber, wavelength and energy deposition combination, efficacy, significant adverse effects, or complications as metrics for comparison.
 Picture of a jacket-tip laser fiber. Courtesy of AngioDynamics (http://www.angiodynamics.com/).
Picture of a jacket-tip laser fiber. Courtesy of AngioDynamics (http://www.angiodynamics.com/).
Target veins
ELA has been successfully and safely used to ablate the great and small saphenous veins, the anterior and posterior accessory great saphenous vein, the superficial accessory saphenous vein, the anterior and posterior circumflex veins of the thigh as well as the thigh extension of the small saphenous vein, including the vein of Giacomini.
ELA has been used to treat long straight competent tributary veins outside the superficial fascia, particularly in patients who are obese and who either sclerotherapy or microphlebectomy would be difficult, time consuming, or prone to side effects.[3]
The selection of candidates for ELA involves a directed history, physical examination, and duplex ultrasound (DUS) examination. The details of the clinical and DUS examination have been discussed in other chapters. Indications for endovenous treatment are listed below.
Symptoms affecting quality of life are as follows:
-
Aching
-
Throbbing
-
Heaviness
-
Fatigue
-
Restlessness
-
Night cramps
-
Pruritus
-
Spontaneous hemorrhage
Skin changes associated with chronic venous hypertension are as follows:
-
Corona phlebectasia, eczema, and pigmentation
-
Lipodermatosclerosis
-
Atrophie blanche
-
Healed or active ulceration
-
Edema
-
Superficial phlebitis (SVT) in varicose veins
Cosmetic (restorative) concerns are indications for treatment.
Anatomical indications are as follows:
-
Significant reflux documented on DUS examination (reflux >0.5 seconds)
-
Straight vein segment
-
Intrafascial or epifascial vein segment meeting other anatomical criteria that can be pushed away from the skin with tumescent anesthetic
-
Reflux responsible for venous hypertension leading to the clinical abnormalitiesAmbulatory patient without contraindication
The contraindications to endovenous treatment are listed below.
-
Patients who are pregnant or breastfeeding (concerns related to anesthetic use and heated blood effluent that may pass through the placenta to the fetus)
-
Obstructed deep venous system inadequate to support venous return after ELA
-
Liver dysfunction or allergy making it impossible to use a local anesthetic (cold saline may be useful as an alternative)
-
Allergy to both amide and ester local anesthetics (cold saline may be an alternative)
-
Severe uncorrectable coagulopathy (ELA is safe with warfarin use if the international normalized ratio is between 2 and 3.) [4]
-
Severe hypercoagulability syndromes (where risk of treatment outweighs potential benefits despite prophylactic anticoagulants)
-
Inability to adequately ambulate after the procedure
-
Sciatic vein reflux
-
Thrombus or synechiae in the vein or tortuous vein making passage of an endovenous device impossible (unless multiple access points are chosen)
Treatment of incompetent superficial truncal veins in patients with previous deep vein thrombosis requires a careful assessment of the adequacy of the patent segments of the deep venous system. It also requires a risk stratification of postprocedural thrombosis. ELA is appropriate if the deep system is adequate enough to support venous drainage and the superficial venous incompetence is responsible for significant symptoms or skin changes. If the patient has an ongoing risk for thrombosis, ELA may still be appropriate if that risk can be sufficiently decreased with prophylactic anticoagulants. If saphenous reflux is seen with venous ulcers with an adequate deep venous system, ELA of the causative veins is necessary to minimize the risk of a recurrent ulceration.
Treatment of competent enlarged superficial venous segments has no proven medical benefit and should not be performed. In some cases, the enlarged vein may be functioning as a re-entry or collateral pathway for another source of reflux or deep vein obstruction. The use of ELA to close incompetent perforating veins has been described, and studies show a benefit in ulcer healing and recurrence.[5, 6]
Tumescent anesthetic, when used in phlebology, describes the use of large volumes of dilute anesthetic solutions that are infiltrated into the perivenous space of the veins to be treated. The rationale behind the use of large volume tumescent anesthesia for ELA include its use as a local anesthetic, its ability to empty the vein to maximize the contact of the thermal device and the vein wall for efficient thermal transfer to the vein wall, and providing a protective heat sink around the treated vein to minimize heating of adjacent structures.
ELA is usually performed with a dilute tumescent anesthetic solution of lidocaine with or without epinephrine in normal saline, often buffered with sodium bicarbonate (a concentration of 0.1% lidocaine is typically used with an average volume of about 5–10 mL/cm of treated vein). This should be delivered with ultrasound guidance into the perivenous space (saphenous sheath) of the vein to be treated. It can be injected either manually or with an infusion pump, such that upon completion of the process the vein is surrounded along its entire treated length with the anesthetic fluid, as demonstrated in the image below.
 Transverse ultrasound image of tumescent anesthetic fluid surrounding centrally located great saphenous vein and laser fiber/sheath.
Transverse ultrasound image of tumescent anesthetic fluid surrounding centrally located great saphenous vein and laser fiber/sheath.
Although the maximum safe dosage of lidocaine using the tumescent technique for venous procedures is not well studied, 35 mg/kg with epinephrine has been reported as safe in the plastic surgical literature.[7] However, the US Food and Drug Administration (FDA)–reviewed circulars accompanying units of lidocaine state a maximum dose of 5 mg/kg without and 7 mg/kg with epinephrine with each use. Toxicity may occur related to the dose of lidocaine and or epinephrine. Care should be used in patients who are likely to be more sensitive to the dose of these drugs, including elderly persons. When using epinephrine, the use of ECG monitoring may be prudent.
Basic equipment and supplies for ELA are listed below.
-
Procedure table that can tilt to Trendelenburg and reverse Trendelenburg
-
DUS with at least a 7.5 MHz transducer
-
Sterile gowns, gloves, masks, drapes, gauze
-
Ultrasound gel, sterile ultrasound probe and cord cover
-
Antiseptic preparation fluid
-
Local anesthetic
-
No. 11 scalpel blade, 18-gauge needle, 15º ophthalmic blade, or punch biopsy device
-
18- to 21-gauge needle for percutaneous entry
-
21- to 25-gauge needle for administration of tumescent anesthesia
-
Syringes
-
Normal saline
-
Compression stockings
A foot pedal controlled tumescent anesthetic injection pump can be used to infuse the perisaphenous anesthetic as an alternative to hand injection. Venous access kits that allow the use of a less traumatic 21-gauge needle to insert a 0.018-in guidewire are useful when accessing small veins but do add expense to the procedure. These kits include a 4 or 5F sheath with a dilator tapered to the 0.018-in guidewire. After the catheter and dilator are inserted, the dilator and 0.018-in guidewire can be removed to allow the placement of a standard 0.035-in guidewire. These micropuncture kits are marketed by a variety of vendors.
Additional materials required to perform ELA include the laser generator and sterile
laser fiber (see list below) and sheath long enough to cross the abnormal venous segment(s), usually included in a kit along with a guidewire. ELA is usually performed by placing a 4 or 5F sheath into the vein to be treated over a 0.035-in guidewire and then, after inserting a laser fiber into the sheath, withdrawing the sheath to expose the fiber tip. The sheaths are manufactured in multiple lengths and generally the sheath chosen is as long as or longer than the segment(s) to be treated. Sheaths that have a ruler imprinted on them make it easiest to monitor the rate at which they are withdrawn. In very straight veins, a laser fiber can be advanced beyond its sheath to the starting point of ablation. Kits are now available with blunt-tip laser fibers to facilitate this. However, advancement through the sheath is recommended in tortuous veins to avoid passing the fiber through the vein wall.
ELA tools
ELA can be performed using any of the following wavelengths. Generators and laser fiber kits for use are marketed by multiple vendors, as follows:
Endovenous laser wavelengths commercially available include:
-
810 nm (AngioDynamics Queensbury, NY)
-
940 nm (Dornier MedTech Americas, Inc, Kennesaw, Ga)
-
980 nm (Biolitec, Inc, East Longmeadow, Mass)
-
1064 nm (Sharplan, Inc., NJ)
-
1320 nm (CoolTouch, Roseville, Calif)
-
1470 nm (Biolitec, Angiodynamics)
Laser ablation has been primarily performed with 810-µm bare-tipped fibers, which are premarked to allow the operator to know when the fiber is tip-to-tip with the end of the sheath and when the laser extends a fixed distance beyond the sheath tip. Although many of the original fibers were bare-tipped, many of the currently used fibers are jacketed with ceramic or metal, which, in theory, may decrease vein wall perforation and increase the effective diameter of the fiber, resulting in a decrease in the power density and changing the fiber from a cutting mode into a coagulation mode.[2] Anecdotally, with similar power settings and pull-back rates, there is less pain and bruising, although the long-term success has not been characterized to see if this results in a trade off in efficacy. Limited data are available that compare the different configurations, but anecdotally it is thought that higher, water-specific wavelengths produce less postprocedure pain with equivalent outcomes.
Access to the target vein should be performed with the patient in the supine position. The use of a reverse Trendelenburg position (feet down) in order to increase pressure in the target vein and increase the likelihood of a successful puncture is advisable, especially with small-diameter veins. Once the sheath and laser fiber are inserted as described below, the patient is positioned flat and then in the Trendelenburg position after positioning the laser fiber at the desired starting location. The Trendelenburg position helps to empty the vein and improve energy transfer from the fiber to the vein wall. This is particularly important at the upper end of the greater saphenous vein (GSV), where the vein diameter is larger and the vein is less susceptible to spasm.
ELA procedure for through the sheath laser fiber kits
- Perform preprocedural DUS for mapping of the venous segments to be treated. Mark the course of the vein(s) to be treated and important anatomical landmarks associated with the ablation on the skin, including the proposed venous access site(s) and deep vein junctions. The access site is ideally at the inferior end of the incompetent segment or segments of the treated vein. In most cases, the entire incompetent segment(s) can be treated with 1 puncture. If microphlebectomy will be performed along with ELA, the veins to be removed should be marked at this time as well.
- Prepare the operative tray and equipment. Aside from the thermal ablation device and a venous access kit, only basic supplies such as gauze, a sterilizing solution, sterile barriers, and the tumescent solution, with delivery syringes and needle and an ultrasound probe cover, are needed.
- Carry out sterile preparation and draping of the leg to be treated. Preprocedural antibiotics are not necessary in almost all circumstances as the procedure is performed sterilely and is considered clean.
- Visualize the access site with DUS. Placing the patient in a reverse Trendelenburg or partly sitting position prior to the venous puncture keeps the vein more distended and may facilitate venous access.
- Anesthetize the access site. Nick the skin just large enough to facilitate entry of the sheath through the skin.
- Insert the access needle into the great saphenous vein (GSV) under sonographic guidance. Use of a 21G puncture set, as discussed previously, is preferred particularly when the target vein is < 4 mm in diameter when supine. Cutdown is rarely needed and usually only if percutaneous access fails.
- Place a 0.035-in guidewire into the vein.
- Confirm intravenous placement with ultrasonography.
- Place the introducer sheath over the wire.
- Position the sheath for ELA to the starting point for ablation. Some physicians typically advance the ELA sheath beyond the starting point and later withdraw it with the laser fiber to the starting spot. The movement of withdrawal helps in to accurately identify the tip and position it at the starting point.
- Remove the wire and its dilator if one is used with the sheath. Check for venous return by aspirating the syringe attached to the sheath and flush. Recognize that the sheath tip may be against the vein wall and may not aspirate freely. Also realize that when flushing, microbubbles of air introduced into the vein may produce an acoustic shadow that may limit the ability to see venous detail and device positions.
- Introduce the laser fiber into the sheath so that the fiber reaches the sheath tip. There is generally a mark on the fiber to show this. Then fix the laser fiber and carefully pull back the sheath to expose about 2–3 cm of fiber. Then withdraw the entire sheath-laser fiber to the ablation starting spot. Fine tune the location of the tip of the laser fiber to just below the superficial epigastric vein, anterior accessory GSV (AAGSV), or other large normal junctional vein for the GSV, and just below the thigh extension junction with the short saphenous vein (SSV) for SSV ablations. Some operators choose to position the laser fiber 1-3 cm below the saphenofemoral junction (SFJ) without consideration of the position of the junctional branches. No data support superiority of any of the above procedures in terms of ablation success, junctional recurrences, or common femoral vein thrombosis post procedure. See the image below.
- Connect the laser fiber to its generator and confirm that the tip is in the correct general location by viewing the visible light aiming beam that can be delivered into the laser fiber tip and visualized through the skin. This is an additional way to ensure that the tip of the laser is being visualized accurately and that the laser connections were made appropriately. If the light is not seen in the expected location, troubleshoot the position of the laser or the connection to the laser to understand why.
- Administer tumescent anesthesia with ultrasound guidance after the patient has been placed into the Trendelenburg position to help drain the vein.
- Place appropriate laser safety goggles on everyone in the procedure room and use other appropriate laser safety measures. Connect the laser fiber to the laser and verify proper laser settings. Setting recommendations vary, but aim to deliver at least 70–80 J/cm length of vein treated: at 14 W this is achieved with a pullback rate of 2 mm/s.
- Set the laser to continuous mode and select the power to be used. Re-verify placement of the laser tip with ultrasound.
- Activate the laser and withdraw the fiber and sheath at the speed that is dependent on the amount of energy you wish to deliver at the power setting selected with the laser in continuous mode. Many operators deliver 70-100 J/cm at 14 W in continuous mode at 810 nm throughout the length of the abnormal vein. For the GSV and AAGSV, the author uses more energy for the first 10-12 cm (140 J/cm) and less as the laser tip progresses lower down the leg (100 J/cm to the knee and 70 J/cm below the knee). This is done to ensure closure of the proximal vein segment just below the deep vein junction, where failure occurs most, and to decrease the risk of nerve injuries lower in the leg. For the SSV, the author uses about 112 J/cm for the first 3-4 cm, then 100 J/cm for the next 3-4 cm, and then 70 J/cm for the remaining vein.
- Stop laser energy delivery at the distal aspect of the vein and place the laser in standby mode.
- Remove the fiber/sheath from the vein. Be sure the entire fiber is removed to exclude the possibility of a fracture of the device intravascularly.
- Record the watts, laser on-time, total joules delivered, and length of the segment treated. Calculate the withdrawal rate and joules delivered per cm to ensure you have reached the targets for successful ablation.
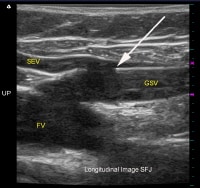 Longitudinal (sagittal) duplex ultrasound image of the saphenofemoral junction during the positioning of the tip of a laser fiber during an endovenous laser ablation. The laser tip is in the greater saphenous vein (GSV) just beyond the superficial epigastric vein (SEV) origin and is marked by the arrow. FV=femoral vein.
Longitudinal (sagittal) duplex ultrasound image of the saphenofemoral junction during the positioning of the tip of a laser fiber during an endovenous laser ablation. The laser tip is in the greater saphenous vein (GSV) just beyond the superficial epigastric vein (SEV) origin and is marked by the arrow. FV=femoral vein.
Technique considerations
The amount of thermal energy delivered is correlated to the success of ELA. With laser, energy deposition has been described as either that deposited per centimeter of vein length (J/cm) or as that deposited to the vein wall using a cylindrical approximation of the inner surface area of the vein (J/cm2), which can be considered a fluence equivalent. Durable vein occlusion was demonstrated in an observational series as more likely when the energy delivered exceeded 80 J/cm with a median observation of 30 weeks.[8] High rates of vein occlusion and ultimate DUS disappearance was noted in a series where the thermal dose in each segment of the GSV was tailored to the diameter in that segment. The ranges of energies used to achieve durable ablation included 50 J/cm for veins ≤4.5 mm and 120 J/cm for veins >10 mm in diameter. No increase in complications was seen with any of the higher energy strategies.[9]
To date, a prospective, randomized evaluation of the relationship of the different variables that can be controlled by the operator on the rate of anatomically successful vein obliteration and complication rates has not been performed. These variables include amount of laser energy deposition (J/cm or J/cm2), the rate of energy deposition (power setting), wavelength, and fiber design. Most combinations of treatments above the 70-80 J/cm seem to result in durable closure and some data support the notion that complications and adverse effects are not increased with energies up to 140 J/cm.
The differences between the current thermal ablation technologies are relatively small. Several retrospective analyses of observational data have demonstrated qualitatively similar occlusion and complication rates with a trend toward quicker treatments and better outcomes with ELA compared with the first generation RFA. In a study comparing Closure Fast (CF) and ELA, equivalent treatment times and anatomical success at 6 months were seen with slightly less immediate postprocedure bruising and postprocedure discomfort noted with CF.[10]
ELA bruising and discomfort have been thought to be less with continuous mode laser deposition than with pulsed mode. Limited data suggest that these side effects may be lessened with the use of a laser fiber with its tip covered with a glass cap and metal sleeve as opposed to a bare fiber. This effectively makes the fiber larger and presumably more coagulating than cutting. The prevention of wall contact produced by the jacket-tipped fibers results in less postprocedure bruising and pain in one study that evaluated 20 patients who were treated with bare-tip fibers and jacket-tip fibers.[11]
Excellent anatomic success of 90-100% occlusion rates has been shown for lasers with wavelengths between 810 and 1470 nm.[12] Recently, the 1470-nm radial laser fiber has been shown to have the lowest amount of postprocedure bruising, pain, parasthesia, induration, and superficial phlebitis due to the water-specific wavelength that is thought to directly act on the vessel wall.[13] Moreover, this study showed equivalent anatomic success of 99.6% closure at 1 year following use of the 1470-nm radial fiber.
Adverse events and complications
Adverse events following ELA occur, but almost all are minor. Ecchymosis over the treated segment frequently occurs and normally lasts for 7-14 days. About one week after ELA, the treated vein may develop a feeling of tightness similar to that after a strained muscle. This transient discomfort, likely related to inflammation in the treated vein segment, is self-limited and may be ameliorated with the use of nonsteroidal anti-inflammatory drugs (NSAIDs), ambulation, stretching, and graduated compression stockings. Both of these side effects are more commonly described after ELA using existing laser protocols than for RFA, but the differences in severity are very small when studied objectively.[10]
Superficial phlebitis is another uncommon side effect of ELA, being reported after about 5% of treatments as mentioned previously. There are no published reports of superficial phlebitis after ELA progressing to deep vein thrombosis and it has been managed in most series with NSAIDs, graduated compression stockings, and ambulation. Anecdotally, superficial phlebitis seems to be more common in larger diameter tributary varicose veins or in varicose veins that have their inflow and outflow ablated by ELA. Concurrent phlebectomy of these veins at the time of ELA has been recommended to decrease the risk of this side effect, but at this point no data substantiate this claim.
More significant adverse events reported following ELA include neurologic injuries, skin burns, and DVT. The overall rate of these complications has been shown to be higher in low-volume centers than high-volume centers. The nerves at highest risk include the saphenous nerve, adjacent to the GSV below the mid-calf perforating vein, and the sural nerve adjacent to the SSV in the mid and lower calf. Both of these nerves have only sensory components. The most common manifestation of a nerve injury is a paresthesia or dysesthesia, most of which is transient. The nerve injuries can occur with the trauma associated with catheter introduction, during the delivery of tumescent anesthesia, or by thermal injury related to heating of the perivenous tissues.
Tumescent anesthesia has been demonstrated to reduce perivenous temperatures with laser and RF ablation. The delivery of the perivenous fluid is felt to be responsible for the low rate of cutaneous and neurologic thermal injuries seen in the series of patients treated using perivenous fluid. Neurologic injuries are seen after truncal vein removal and are related to injury to nerves adjacent to the treated vein. The incidence of these adverse events are related to the degree to which objective testing is performed to identify them. In general, paresthesias caused by ELA are usually temporary with the rate of permanent paresthesias typically reported for GSV and SSV as 0–10%.
The one-week paresthesia rate following RFA was shown to decrease from 15% to 9% after the introduction of tumescent anesthesia. Patients treated with laser ELA performed without tumescent anesthetic infiltrations also demonstrated a high rate of such injuries. Evidence suggests a higher rate of nerve injuries when treating the below knee GSV as compared with the above knee segment and the SSV. Treatment of the below knee GSV or lower part of the SSV may be necessary in many patients to treat to eliminate symptoms or skin disease caused by reflux to the ankle.
A retrospective review demonstrated that below knee laser ablation can be performed with an 8% rate of mild but permanent paresthesias with adequate amounts of tumescent anesthesia. This data also suggests that sparing the treatment of the distal 5–10 cm may have clinical benefit and reduce saphenous nerve injury risk in patients with reflux to the medial malleolus. Skin burns following ELA have been reported. Skin burns are fortunately relatively rare and seem to be avoidable with adequate tumescent anesthesia. The rate of skin burn in 1 series using RFA was 1.7% before and 0.5% after the initiation of the use of tumescent technique during RFA. The early experience had rates as high as 4% that decreased to almost 0% as the use of tumescent anesthesia became a standard of practice.
DVT following ELA is unusual. DVT can occur as an extension of thrombus from the treated truncal vein across the junctional connection into the femoral or popliteal veins. The reported rates of junctional thrombosis following GSV ELA varies widely. This variability may relate to the time of the follow-up examination and the methods used. Most published series using early DUS (around 72 hours or less after ELA) document a proximal extension for the GSV around 1%.
The risk of venous thromboembolism (VTE) is higher in patients with a history of prior DVT or phlebitis, CEAP (clinical, etiological, anatomical and pathological) classification of 3 or greater, and male sex.[14] Endothermal heat-induced thrombosis (EHIT) defines the extent of superficial thrombosis and its extension into the deep venous system as proposed by Kabnick.[14] EHIT 1 represents thrombus up to the SFJ, EHIT 2 represents thrombus extending into the femoral vein occupying less than 50% of luminal diameter, EHIT 3 represents thrombus occupying greater than 50% of femoral vein luminal diameter, and EHIT 4 represents occlusive thrombus in the femoral vein. EHIT 1 is treated conservatively. If identified, EHIT 2 is usually treated with anticoagulation (full or prophylactic intensity are both used), although some advocate early re-examination and conservative care for more minor forms. EHIT 3 and 4, which are much less common, probably merit full anticoagulation.
Those performing the DUS at a later interval identify a lower rate of EHIT. Possibly, the rates are different for different operators with different protocols or the proximal extension of thrombus may be self-limited and may resolve by 1 month without a clinical event. Pooling data from several sources suggest that the incidence is approximately 0.3% after ELA. This type of DVT is almost universally asymptomatic. The significance of this type of thrombus extension into the femoral vein seems to be different from that found with native GSV thrombosis with extension or when compared with typical femoral vein thrombosis.
The incidence of junctional extension of thrombus after SSV ablation has also been described to be low (0-6%). In one study, the rate of popliteal extension of SSV thrombus at 2-4 days after ELA was related to the anatomy of the SPJ. The incidence at 48-72 hours of follow-up was 0% when no SPJ existed, 3% when a thigh extension exists, but 11% when no thigh extension could be identified just above the SPJ. Heparin was used to treat identified thrombus extensions and all regressed. No published data are available on conservative management of transjunctional thrombus extension at either the SPJ or SFJ. However, given that popliteal or femoral vein obstruction develops in significantly less than 1% of patients, including in those series where DUS is not done until 1 month after ELA, the practice of performing early DUS surveillance and aggressive anticoagulation of such findings is controversial.
Neovascularity at the SFJ after ELA, as a form of recurrence of varicose veins, seems to be rare at 1- to 3-year follow-up. Neovascularization was seen in only 2 of the 1222 limbs followed for up to 5 years in an industry-sponsored registry of patients treated with RFA. Longer follow-up may be necessary to feel confident with this observation. However, neovascularization is common and often an early event following high-ligation and stripping (HL/S). Neovascularization may be less common following endovenous procedures because the junctional tributary flow, which was usually ligated at their confluence with the SFJ, is generally not affected with GSV ELA.
Anecdotal reports of laser fiber fracture or retained venous access sheaths have been made to the device manufacturers and a case report exists describing a retained vascular sheath after laser ablation. Respecting the fragile glass laser fibers and being gentle with its handling should help minimize laser fiber fractures. The possibility of a laser fiber fracture should be considered with the removal of the device in each case. Care to deliver thermal energy only beyond the introducer sheath and away from any other parallel placed sheaths when treating 2 veins during the same procedure is essential to avoid severing segments of these catheters. No specific management recommendations of retained intravenous laser fiber or sheath fragments can be made based on the data. However, anecdotally, retained short segments of the distal end of the laser fiber seem to be well tolerated without incident and efforts to remove them may be more prone to adverse events than managing them conservatively.
A case report of an arteriovenous fistula (AVF) between a small popliteal artery branch near the SPJ and the SSV exists. Anecdotal references have been made of additional AVFs between the proximal GSV and the contiguous superficial external pudendal artery. Although thought to be related to a heat-induced injury caused by the thermal device, an AVF could be caused by a needle injury during tumescent anesthetic administration. Ways to minimize the risk of these AVFs include careful advancement of the intravascular devices, atraumatic delivery of the tumescent anesthetic, the use of copious amounts of tumescent fluid, and avoidance of treating the subfascial portion of the SSV where popliteal artery branches exist adjacent to the SSV.
Postoperative Care and Instructions
Postoperative care is designed to improve efficacy and minimize side effects and the risk of complications. There is a diversity of opinion about what is necessary as no evidence supports any specific recommendations. Immediately postoperatively, almost all physicians recommend some form of compression. The most common recommendation is for class II compression stockings (30–40 mm Hg) applied immediately after the procedure and worn for 1–2 weeks. The clinical value of this practice is not substantiated by data. Anecdotally, patients feel better with the use of compression, especially during the second week when the pulled-muscle feeling occurs.
Patients are encouraged to ambulate for at least 30–60 minutes after leaving the procedure room and at least 1–2 hours daily for 1–2 weeks. Hot baths, running, jumping, heavy lifting, and straining are discouraged by many physicians for 1–2 week. NSAIDs may be taken on an as-needed basis for discomfort.
Patients are generally seen at 1 month after the procedure to assess the results by clinical examination and DUS. Some physicians recommend a follow-up DUS 24–72 hours after the procedure as surveillance for junctional thrombus extension from the treated vein into the contiguous deep vein. However, as mentioned, the yield of this early examination for identifying extension of thrombus beyond the deep junction extending into the femoral vein for GSV or popliteal vein for SSV ablation is at most 1%. Moreover, treatment of such nonocclusive extensions is controversial and increasingly conservative care is recommended. Most physicians agree that repeat DUS at about 9-12 months after the procedure ultimately determines the anatomical success of the ablation.
Results of ELA
General comments
ELA is safely and effectively performed using local anesthesia in an office setting requiring about 45–90 minutes of room time to be performed. Procedure times are dependent on the number of concurrent treated veins, length of segment(s) treated, and whether ancillary procedures, such as ambulatory phlebectomy, are carried out. Patient satisfaction has been reported to be very high.
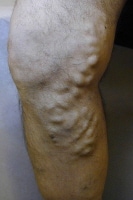 Varicose vein before treatment with endovenous laser therapy.
Varicose vein before treatment with endovenous laser therapy.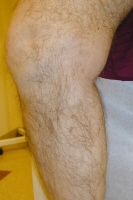 Varicose vein after treatment with endovenous laser.
Varicose vein after treatment with endovenous laser.
The total cost (cost of the procedure plus societal cost) of endovenous procedures is likely equal to or better than that of surgery. This is debatable in a hospital setting, but is almost certainly true if the ELA can be performed in a nonspecialized office setting. These techniques are being rapidly adopted and are now being performed more often than traditional HL/S in the United States.
Anatomical success rates
The anatomical outcomes following endovenous treatment include occlusion of the treated segment, early failure (complete or segmental), or late recanalization (complete or segmental). Anatomic success following ELA should result in the treated vein having no lumen and either shrink to a fibrous cord < 2.5 mm in diameter or become sonographically absent 6–12 months after treatment. Anatomical success with ELA of the GSV has been reported between 93–100%. The follow-up for these evaluations varies from 3 months to 4 years.
What is Varicose Vein Treatment (Endovenous Ablation of Varicose Veins)?
Varicose vein treatment, or endovenous ablation, is a minimally invasive treatment that uses radiofrequency or laser energy to cauterize (burn) and close abnormally enlarged veins in the legs, a condition called varicose veins.
Normally, blood circulates from the heart to the legs via arteries and back to the heart through veins. Veins contain one-way valves which allow blood to return from the legs against gravity. If the valves leak, blood pools in leg veins which can become enlarged or varicose.
Endovenous ablation is an image-guided procedure that uses heat generated by radiofrequency or laser energy to seal off these faulty vessels, diverting blood flow immediately to nearby healthy veins.
What are some common uses of the procedure?
Although this procedure may be used for cosmetic purposes, it is more commonly used to help alleviate symptoms. Symptoms are typically due to enlarged nonfunctional veins that cause circulatory problems (venous insufficiency). Leg symptoms can include:
How should I prepare?
You should report to your doctor all medications that you are taking, including herbal supplements, and if you have any allergies, especially to local anestheticmedications, general anesthesia or to contrast materials containing iodine (sometimes referred to as "dye" or "x-ray dye"). Your physician may advise you to stop taking aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs) or blood thinners for a specified period of time before your procedure.
You should wear comfortable, loose-fitting clothing. You will need to remove all clothing and jewelry in the area to be examined.
You should plan to have a relative or friend drive you home after your procedure.
You may be asked to wear a gown during the procedure.
What does the equipment look like?
In this procedure, an ultrasound machine, catheter, laser fiber or radiofrequency electrode, and laser or radiofrequency console are used.
Ultrasound scanners consist of a console containing a computer and electronics, a video display screen and a transducer that is used to do the scanning. The transducer is a small hand-held device that resembles a microphone, attached to the scanner by a cord. The transducer sends out inaudible high frequency sound waves into the body and then listens for the returning echoes from the tissues in the body. The principles are similar to sonar used by boats and submarines.
The ultrasound image is immediately visible on a video display screen that looks like a computer or television monitor. The image is created based on the amplitude (loudness), frequency (pitch) and time it takes for the ultrasound signal to return from the area of the patient being examined to the transducer (the device used to examine the patient), as well as the type of body structure and composition of body tissue through which the sound travels. A small amount of gel is put on the skin to allow the sound waves to travel back and forth from the transducer.
A catheter is a long, thin plastic tube that is considerably smaller than a "pencil lead", or approximately 1/8 inch in diameter.
Laser fibers (fiber optics) or radiofrequency electrodes carry laser or electrical energy from their respective power generators into the body.
How does the procedure work?
Using ultrasound to visualize the enlarged vein, a catheter or vascular access sheath is inserted through the skin and positioned within the abnormal vein. A laser fiber or radiofrequency electrode is then inserted through the catheter and advanced to the desired location. Laser or radiofrequency energy is then applied, heating the vessel and causing it to close. Following the procedure, the faulty vein will shrink and "scar down."
How is the procedure performed?
Image-guided, minimally invasive procedures such as endovenous ablation are performed by a specially trained interventional radiologist.
This procedure is often done on an outpatient basis. However, some patients may require admission following the procedure. Please consult with your physician as to whether or not you will be admitted.
Your physician may use a topical anesthetic cream over the abnormal vein area before the procedure in order to reduce discomfort.
Your physician will numb the area where the catheter will enter into the abnormal vein with a local anesthetic and use the ultrasound transducer or wand to study the vein and track its path.
The leg being treated will be cleaned, sterilized and covered with a surgical drape.
A very small skin incision is made at the site.
Using ultrasound guidance, the catheter is inserted through the skin into the vein and positioned within the abnormal vein. The laser fiber or radiofrequency electrode is inserted through the catheter and the tip of the fiber or electrode is exposed by pulling the catheter back slightly.
Local anesthetic is injected around the abnormal vein with ultrasound guidance. Laser or radiofrequency energy is applied as the catheter is slowly withdrawn.
Pressure will be applied to prevent any bleeding and the opening in the skin is covered with a bandage. No sutures are needed.
This procedure is usually completed within an hour.
What will I experience during the procedure?
You will be asked to wear protective glasses if and when lasers are in use.
An area of your leg will be cleaned, shaved and numbed.
You will feel a slight pin prick when the local anesthetic is injected.
The area will become numb within a short time.
You may feel slight pressure when the catheter is inserted, but no serious discomfort.
Injection of local anesthetic around the abnormal vein is the most bothersome part of the procedure because it usually requires multiple injections along the vein. Actual closure of the vein with laser or radiofrequency is usually not painful.
Following the procedure, you will need to wear a gradient compression stocking to help reduce bruising, tenderness, and minimize the rare possibility that blood clots may form.
You may resume your normal activity immediately, with the exception of air travel or prolonged sitting (such as a long car trip). You should remain active and not spend too much time in bed during the recovery period since this increases the chance of complications.
Who interprets the results and how do I get them?
A follow up ultrasound examination is essential in order to assess the treated vein and to check for adverse outcomes. Within one week, the target vein should be successfully closed. Additional procedures (such as sclerotherapy or phlebectomy) may be necessary to treat associated veins.
What are the benefits vs. risks?
Benefits
- No surgical incision is needed—only a small nick in the skin that does not have to be stitched closed.
- When compared with traditional vein stripping techniques, endovenous ablation is more effective, has fewer complications, and is associated with much less pain during recovery.
- Endovenous ablation is generally complication-free and safe.
- This procedure leaves virtually no scars because catheter placement requires skin openings of only a few millimeters, not large incisions.
- Endovenous ablation offers a less invasive alternative to standard surgery.
- Most of the veins treated are effectively invisible even to ultrasound 12 months after the procedure.
- Most patients report symptom relief and are able to return to normal daily activities immediately, with little or no pain.
Risks
- Any procedure where the skin is penetrated carries a risk of infection. The chance of infection requiring antibiotic treatment appears to be less than one in 1,000.
- Any procedure that involves placement of a catheter inside a blood vessel carries certain risks. These risks include damage to the blood vessel, bruising or bleeding at the puncture site, and infection.
- Some postoperative bruising and tenderness may occur, but may be alleviated by wearing a compression stocking.
- Some instances of thermal (heat) damage to nerves have been reported. This is rare and generally goes away in a short time.
- Thrombophlebitis (inflammation of the vein) is not uncommon and may cause pain and redness over the treated area, but generally responds well to nonsteroidial anti-inflammatory drugs (NSAIDs). Blood clots that formed in the veins can travel to the lungs (pulmonary embolism); however, this is an extremely rare occurrence.
What are the limitations of Endovenous Ablation of Varicose Veins?
Ablation catheters cannot be easily passed through a tortuous vein, or a vessel with many turns and bends. Consequently, the procedure is typically used to treat larger varicose veins, such as the great saphenous vein, which extends from the groin down the inside of the thigh into the inner calf.
Endovenous ablation is successful at closing the abnormal target vein almost 100 percent of the time, but small dilated branches that persist in the skin often require additional treatment with phlebectomy (minor surgical procedure to extract them) or sclerotherapy (injection of a liquid medication to seal them off). Subsequent treatments are usually scheduled after an ablation procedure.
Varicose veins and spider veins facts
- Veins carry blood low in oxygen content from the body to the lungs and heart.
- Varicose veins can lead to aching or even ulceration of the legs.
- Varicose veins can be caused be weakened valves in the veins or weakened walls of the veins, or by inflammation in the veins (phlebitis).
- Varicose veins and spider veins are not dangerous (with rare exceptions).
- Interventions and treatments such as surgery, "ablation" by laser, radiofrequency or other technology is necessary in settings where veins cause significant symptoms that do not respond to non-interventional treatment.
- Treatments available for venous disease include surgery and sclerotherapy, among other techniques.
What are veins and what is their function?
Veins are blood vessels that return blood from all the organs in the body toward the heart. When the different organs use oxygen from the blood to perform their functions, they release the used blood containing waste products (such as carbon dioxide) into the veins. Blood in the veins is then transported to the heart and returned to the lungs, where the waste carbon dioxide is released and more oxygen is loaded by the blood and taken back to the rest of the body by the arteries.
Veins also act as a storage for unused blood. When the body is at rest, only a portion of the available blood in the body circulates. The rest of the blood remains inactive in the veins and enters the active circulation when the body becomes more active and needs the additional blood to carry oxygen to the entire body. This storing capacity is due to the elasticity (flexibility to expand) of the walls of the veins.
Veins have different sizes that depends their location and function. The largest veins are in the center of the body; these collect the blood from all the other smaller veins and channel it into the heart. The branches of these large veins get smaller and smaller as they move away from the center of the body. The veins closer to the skin surface are called superficial veins. The veins that are deeper and closer to the center of the body are called deep veins. There are also other veins that connect the superficial veins to the deep veins, and these are called perforating veins.