 |
|---|
หน้าหลัก | สุขภาพดี | สุภาพสตรี | การแปลผลเลือด | โรคต่างๆ | วัคซีน
เมื่อใช้ความร้อนน้ำมันมะกอก และน้ำมันคาโนล่าจะเกิดสาร Aldehydes น้อยกว่าน้ำมันดอกทานตะวันและน้ำมันข้าวโพด 20 เท่า สาร Aldehydes นี้จะเป็นสารที่ทำให้เกิดมะเร็งไม่ว่าจะเกิดจากการสูดดมหรือการรับประทาน
การใช้น้ำมันอย่าปลอดภัย
สารพิษที่เกิดจากการใช้น้ำมัน
ไขมันไม่อิ่มตัวเชิงซ้อน(หลายตำแหน่ง)มีประโยชน์ในการลดไขมันในเลือดและป้องกันโรคหัวใจ แต่หากเราเก็ยไขมันให้ถูกแสงึ่งทำให้เกิดสารพิษทีละน้อย แต่หากทำอาหารอุณหภูมิมากกว่าจุดเกิดควันก็จะเกิดสารซึ่งทำให้เกิดโรคมะเร็งและหลอดเลือดหัวใจ ส่วนไขมันอิ่มตัวจะทนความร้อนได้ดี แต่เนื่องจากเป็นไขมันิ่มตัวหากรับประทานมากจะทำให้เกิดโรคหัวใจและหลอดเลือด แต่เกิดสารพิษน้อยเมื่อเจอความร้อนสูง
ส่วนไขมันไม่อิ่มตัวเชิงเดี่ยว(หนึ่งตำ)แหน่งหากเป็น extra virgin หรือ virgin หากรับประทานสดจะมีคุณค่าทางอาหารสูง และคุณค่าทางอาหารจะลดลงเมื่อเจอความร้อน แต่เกิดสารพิษไม่มาก
โดยสรุป
RANCIDIFICATION |
|
| When oils and fats are exposed to warm and moist air for a long time they give off offensive odor and become off-tastes. This process is called Rancidification. Rancidification caused by the hydrolysis of ester linkage or oxidation at double bond forming volatile aldehydes or bad smell. | |
It can become rancid:
Cooking food in fat or oil changes the overall PUFA content in several ways:
The degree and complexity of the changes depend on the cooking method. Frying causes the largest changes because of the high temperatures involved and the interactions that take place between the food and the cooking oil. Fats and oils that are high in saturated fatty acids and monounsaturated acids are more suitable for frying because they are more stable when heated. Use coconut oil, palm oil, butter, cocoa butter, olive oil or canola oil. From a PUFA perspective, frying is much more complex than baking or grilling because:
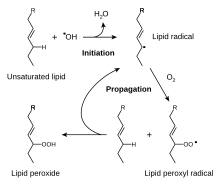
Apart from the issue of instability described above, research has shown that the type of cooking oil used directly affects the ultimate PUFA content of the cooked food. Deep-frying in olive oil enriches the food in oleic acid while deep-frying in sunflower oil enriches the food in LA, therefore impacting the ω-3 to ω-6 ratio adversely. Choose your cooking oil with care: Frying foods in corn, sunflower, safflower or soya oil enriches the food with LA, resulting in an adverse ω-3 to ω-6 ratio. Choose palm oil, butter, olive oil, canola oil, rapeseed oil or coconut oil to help maintain a more healthy PUFA balance.4 You can improve your ω-3 to ω-6 balance by using canola, rapeseed, olive or palm oil instead of oils that have a much higher LA content. Although coconut oil has no ALA, it has one of the lowest LA contents and has other advantages, including very good heat stability and high resistance to rancidity due to its high saturated fat content. Canola and rapeseed oil have the best ω-3 to ω- 6 ratios. Olive oil and palm oil are even lower in LA content although they contain very little ALA and hence have a less well balanced ω-3 to ω-6 ratio. Lipid peroxidation refers to the oxidative degradation of lipids. It is the process in which free radicals "steal" electrons from the lipids in cell membranes, resulting in cell damage. This process proceeds by a free radical chain reaction mechanism. It most often affects polyunsaturated fatty acids, because they contain multiple double bonds in between which lie methylene bridges (-CH2-) that possess especially reactive hydrogens. As with any radical reaction, the reaction consists of three major steps: initiation, propagation, and termination. Initiation is the step in which a fatty acid radical is produced.
| Cooking oils | Cooking Oils α-Linolenic % by weight of total Fatty Acids | Linoleic % by weight of total Fatty Acids | n-3 to n-6 Ratio |
| Coconut Oil | 0.0 | 1.5 | NA |
| Canola Oil | 9.2 | 18.7 | 1 to 2.0 |
| Rapeseed Oil | 7.3 | 14.6 | 1 to 2.0 |
| Soybean Oil | 7.8 | 53.2 | 1 to 6.8 |
| Olive Oil | 0.6 | 10.0 | 1 to 16.7 |
| Palm Oil | 0.3 | 9.4 | 1 to 31.3 |
| Corn Oil | 0.9 | 57.0 | 1 to 63.3 |
| Sunflower Oil | 0.5 | 68.2 | 1 to 136.4 |
| Cottonseed Oil | 0.3 | 53.3 | 1 to 177.7 |
| Sesame Oil | 0.2 | 43.3 | 1 to 216.5 |
| Peanut Oil | None | 31.4 | NA |
| Safflower Oil | None | 77.7 | NA |
The most notable initiators in living cells are reactive oxygen species (ROS), such as OH· and HO2, which combines with a hydrogen atom to make water and a fatty acid radical. The fatty acid radical is not a very stable molecule, so it reacts readily with molecular oxygen, thereby creating a peroxyl-fatty acid radical. This radical is also an unstable species that reacts with another free fatty acid, producing a different fatty acid radical and a lipid peroxide, or a cyclic peroxide if it had reacted with itself. This cycle continues, as the new fatty acid radical reacts in the same way. When a radical reacts with a non-radical, it always produces another radical, which is why the process is called a "chain reaction mechanism". The radical reaction stops when two radicals react and produce a non-radical species. This happens only when the concentration of radical species is high enough for there to be a high probability of collision of two radicals. Living organisms have different molecules that speed up termination by catching free radicals and, therefore, protecting the cell membrane. One important such antioxidant is vitamin E. Other anti-oxidants made within the body include the enzymes superoxide dismutase, catalase, and peroxidase. The end products of lipid peroxidation are reactive aldehydes, such as malondialdehyde (MDA) and 4-hydroxynonenal (HNE), the second onebeing known also as "second messenger of free radicals" and major bioactive marker of lipid peroxidation, due to its numerous biological activities resembling activities of reactive oxygen species.5 How to slow down rancidity in cooking oils? To slow rancidity from hydrolysis and oxidation: •store cooking oils in dark or covered/wrapped bottles away from direct sunlight, or in metal tins with the oil nearing the top of the tin •store cooking oils in cool and dark places – best refrigerated (keep in mind that highly monounsaturated oils, like olive oil will become semi-solid at low temperatures) •use lower cooking temperatures (with the right cooking oils – below) and DO NOT reuse oils for cooking or frying! •in commercial applications, with the use of natural (polyphenols, ascorbic acid, mixed tocopherols) or synthetic antioxidants, added to oils. What oils should you use for cooking?
The best oils for cooking are those that are highly unsaturated or primarily monounsaturated in this order:
wikipedia
Rancidification, the product of which can be described as rancidity, is the process which causes a substance to become rancid, that is, having a rank, unpleasant smell or taste. Specifically, it is the hydrolysis and/or autoxidation of fats into short-chain aldehydes and ketones which are objectionable in taste and odor.[1] When these processes occur in food, undesirable odors and flavors can result. In some cases, however, the flavors can be desirable (as in aged cheeses).[2] In processed meats, these flavors are collectively known as warmed-over flavor. Rancidification can also detract from the nutritional value of food, and some vitamins are highly sensitive to degradation.[3] Akin to rancidification, oxidative degradation also occurs in other hydrocarbons, e.g. lubricating oils, fuels, and mechanical cutting fluids.[4]
Rancidification pathways[edit]
Three pathways for rancidification are recognized:[5]
Hydrolytic rancidity[edit]
Hydrolytic rancidity refers to the odor that develops when triglycerides are hydrolyzed and free fatty acids are released. This reaction of lipid with water sometimes requires a catalyst, but results in the formation of free fatty acids and salts of free fatty acids. In particular, short-chain fatty acids, such as common butter fats, are odorous. Rancidity in foods may be very slight, indicated by a loss of freshness to very severe, indicated by objectionable odors and/or flavors.
Oxidative rancidity[edit]
Oxidative rancidity is associated with the degradation by oxygen in the air. Via a free radical process, the double bonds of an unsaturated fatty acid can undergo cleavage, releasing volatile aldehydes and ketones. Oxidation primarily occurs with unsaturated fats. For example, even though meat is held under refrigeration or in a frozen state, the poly-unsaturated fat will continue to oxidize and slowly become rancid. The fat oxidation process, potentially resulting in rancidity, begins immediately after the animal is slaughtered and the muscle, intra-muscular, inter-muscular and surface fat becomes exposed to oxygen of the air. This chemical process continues during frozen storage, though more slowly at lower temperature. The process can be suppressed by the exclusion of oxygen or by the addition ofantioxidants.[citation needed] Thus, airtight packaging will slow rancidity development.t
Microbial rancidity[edit]
Microbial rancidity refers to a process in which microorganisms, such as bacteria or molds, use their enzymes such as lipases to break down fat. This pathway can be prevented by sterilization.
Health effects[edit]
Consuming rancid food products is unlikely to cause immediate illness or harm. Rancidification can reduce the nutritional value of food, and some vitamins are highly sensitive to degradation.[3] In addition, rancidification can produce potentially toxic compounds associated with long-term harmful health effects concerning advanced aging, neurological disorders, heart disease, and cancer.[6]
Reducing rancidification[edit]
Antioxidants are often used as preservatives in fat-containing foods to delay the onset or slow the development of rancidity due to oxidation. Natural antioxidants include polyphenols (for instance flavonoids), ascorbic acid (vitamin C) and tocopherols (vitamin E). Synthetic antioxidants include butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), TBHQ, propyl gallate and ethoxyquin. The natural antioxidants tend to be short-lived[citation needed], so synthetic antioxidants are used when a longer shelf-life is preferred. The effectiveness of water-soluble antioxidants is limited in preventing direct oxidation within fats, but is valuable in intercepting freeradicals that travel through the aqueous parts of foods. A combination of water-soluble and fat-soluble antioxidants is ideal, usually in the ratio of fat to water.
In addition, rancidification can be decreased, but not completely eliminated, by storing fats and oils in a cool, dark place with little exposure to oxygen or free radicals, since heat and light accelerate the rate of reaction of fats with oxygen. Antimicrobial agents can also delay or prevent rancidification by inhibiting the growth of bacteria or other micro-organisms that affect the process.[1]
Oxygen scavenging technology can be used to remove oxygen from food packaging and therefore prevent oxidative rancidification.
Measurement of oxidative stability[edit]
Oxidative stability is a measure of an oil or fat's resistance to oxidation. Because the process takes place through a chain reaction, the oxidation reaction has a period when it is relatively slow, before it suddenly speeds up. The time for this to happen is called the "induction time", and it is repeatable under identical conditions (temperature, air flow, etc.). There are a number of ways to measure the progress of the oxidation reaction. One of the most popular methods currently in use is the Rancimat method.
The Rancimat method is carried out using an air current at temperatures between 50 and 220 °C. The volatile oxidation products (largely formic acid[7]p. 47) are carried by the air current into the measuring vessel, where they are absorbed (dissolve) in the measuring fluid (distilled water). By continuous measurement of the conductivity of this solution, oxidation curves can be generated. The cusp point of the oxidation curve (the point where a rapid rise in the conductivity starts) gives the induction time of the rancidification reaction,[8] and can be taken as an indication of the oxidative stability of the sample.
The Rancimat method, the oxidative stability instrument (OSI) and the oxidograph were all developed as automatic versions of the more complicated AOM (active oxygen method), which is based on measuring peroxide values,[8] for determining the induction time of fats and oils. Over time, the Rancimat method has become established, and it has been accepted into a number of national and international standards, for example AOCS Cd 12b-92 and ISO 6886.
Smoking Points of Fats and Oils:
Based on the above classification, the ideal cooking oil should contain higher amounts of monounsaturated and polyunsaturated fats, with a minimal or no saturated fats and trans fats. Different fats and oils have different uses. Each performs best within a certain range of temperature. Some are made for high heat cooking, while others have intense flavors that are best enjoyed by drizzling directly on food.
The smoke point of an oil or fat is the temperature at which it gives off smoke. The smoke point of oil depends to a very large extent on its purity and age at the time of measurement. A simple rule of thumb is that the lighter the color of the oil, the higher its smoke point. When frying, it is important to choose an oil with a very high smoking point. Most foods are fried between the temperatures of 350-450 degrees Fahrenheit so it is best to choose an oil with a smoking point above 400 degrees.
Fats or Oils |
Description |
Cooking Uses |
Type of Fat |
Smoke Point °F |
Smoke Point °C |
Almond Oil |
|
|
Monounsaturated |
420°F |
216°C |
Avocado Oil |
|
Stir frying, searing |
Monounsaturated |
520°F |
271°C |
Butter |
|
Baking, cooking |
Saturated |
350°F |
177°C |
Butter (Ghee), clarified |
|
Frying, sautéing |
Saturated |
375-485°F (depending on purity) |
190-250°C (depending on purity), |
|
A light, golden-colored oil. |
Good all-purpose oil. Used in salads and cooking. |
Monounsaturated |
400°F |
204°C |
Coconut Oil |
|
coatings, confectionary, shortening |
Saturated |
350°F |
177°C |
Corn Oil |
|
Frying, salad dressings, shortening |
Polyunsaturated |
450°F |
232°C |
Cottonseed Oil |
|
|
Polyunsaturated |
420°F |
216°C |
Grapeseed Oil |
Light, medium-yellow oil that is a by-product of wine making. |
|
Polyunsaturated |
392°F |
200°C |
Hazelnut Oil |
|
Salad dressings, marinades and baked goods. |
Monounsaturated
|
430°F |
221°C |
Lard |
|
Baking and frying |
Saturated |
370°F |
182 °C |
Macadamia Nut Oil |
|
Sauté, pan fry, sear, deep fry, stir fry, grill, broil, baking. |
Monounsaturated
|
390°F |
199 °C |
Olive Oil |
|
cooking, salad dressings, sauté, pan fry, sear, deep fry, stir fry, grill, broil, baking |
Monounsaturated |
Extra Virgin -320°F |
160°C |
Palm Oil |
|
Cooking, flavoring |
Saturated |
446°F |
230°C |
Peanut Oil |
|
Frying, cooking, salad dressings |
Monounsaturated |
450°F |
232°C |
Rice Bran Oil |
|
Frying, sauté, salad dressings, baking, dipping oils |
Monounsaturated |
490°F |
254°C |
Safflower Oil |
|
Margarine, mayonnaise, salad dressings |
Polyunsaturated |
450°F |
232°C |
Sesame Oil |
|
Cooking, salad dressings |
Polyunsaturated |
410°F |
232°C |
Shortening, Vegetable |
|
Baking, frying |
Saturated |
360°F |
182 °C |
Soybean Oil |
|
Margarine, salad dressings, shortening |
Polyunsaturated |
450°F |
232°C |
Sunflower Oil |
|
Cooking, margarine, salad dressings, shortening |
Polyunsaturated |
450°F |
232°C |
Vegetable Oil |
|
Cooking, salad dressings |
Polyunsaturated |
|
|
Walnut Oil |
|
Sauté, pan fry, sear, deep fry, stir fry, grill, broil
|
Monounsaturated |
400°F |
204°C |
Sources:
Harvard School of Public Health.
Hormel Foods.
Spectrum Oils.
เอกสารอ้างอิง
| Fat | Smoke Point °F | Smoke Point °C |
|---|---|---|
| Unrefined canola oil | 225°F | 107°C |
| Unrefined flaxseed oil | 225°F | 107°C |
| Unrefined safflower oil | 225°F | 107°C |
| Unrefined sunflower oil | 225°F | 107°C |
| Unrefined corn oil | 320°F | 160°C |
| Unrefined high-oleic sunflower oil | 320°F | 160°C |
| Extra virgin olive oil | 320°F | 160°C |
| Unrefined peanut oil | 320°F | 160°C |
| Semirefined safflower oil | 320°F | 160°C |
| Unrefined soy oil | 320°F | 160°C |
| Unrefined walnut oil | 320°F | 160°C |
| Hemp seed oil | 330°F | 165°C |
| Butter | 350°F | 177°C |
| Semirefined canola oil | 350°F | 177°C |
| Coconut oil | 350°F | 177°C |
| Unrefined sesame oil | 350°F | 177°C |
| Semirefined soy oil | 350°F | 177°C |
| Vegetable shortening | 360°F | 182°C |
| Lard | 370°F | 182°C |
| Macadamia nut oil | 390°F | 199°C |
| Refined canola oil | 400°F | 204°C |
| Semirefined walnut oil | 400°F | 204°C |
| High quality (low acidity) extra virgin olive oil | 405°F | 207°C |
| Sesame oil | 410°F | 210°C |
| Cottonseed oil | 420°F | 216°C |
| Grapeseed oil | 420°F | 216°C |
| Virgin olive oil | 420°F | 216°C |
| Almond oil | 420°F | 216°C |
| Hazelnut oil | 430°F | 221°C |
| Peanut oil | 440°F | 227°C |
| Sunflower oil | 440°F | 227°C |
| Refined corn oil | 450°F | 232°C |
| Palm oil | 450°F | 232°C |
| Palm kernel oil | 450°F | 232°C |
| Refined high-oleic sunflower oil | 450°F | 232°C |
| Refined peanut oil | 450°F | 232°C |
| Refined Safflower oil | 450°F | 232°C |
| Semirefined sesame oil | 450°F | 232°C |
| Refined soy oil | 450°F | 232°C |
| Semirefined sunflower oil | 450°F | 232°C |
| Olive pomace oil | 460°F | 238°C |
| Extra light olive oit | 468°F | 242°C |
| Soybean oil | 495°F | 257°C |
| Safflower oil | 510°F | 266°C |
| Avocado oil | 520°F | 271°C |
I like cooking with extra light olive oil and butter. This is mainly because olive oil is high in monounsaturated fatty acids (73%) while being low in polyunsaturated fatty acids (less than 10%). The refined nature of extra light olive oil mainly affects taste and smoke point, but does not reduce the nutritional benefits of olive oil. Butter, although high in saturated fat (66%), is low in polyunsaturated (4%) and contains a host of vitamins, antioxidants, essential fatty acids, and acids that are antimicrobial and antitumorigenic. Also, it tastes good.
